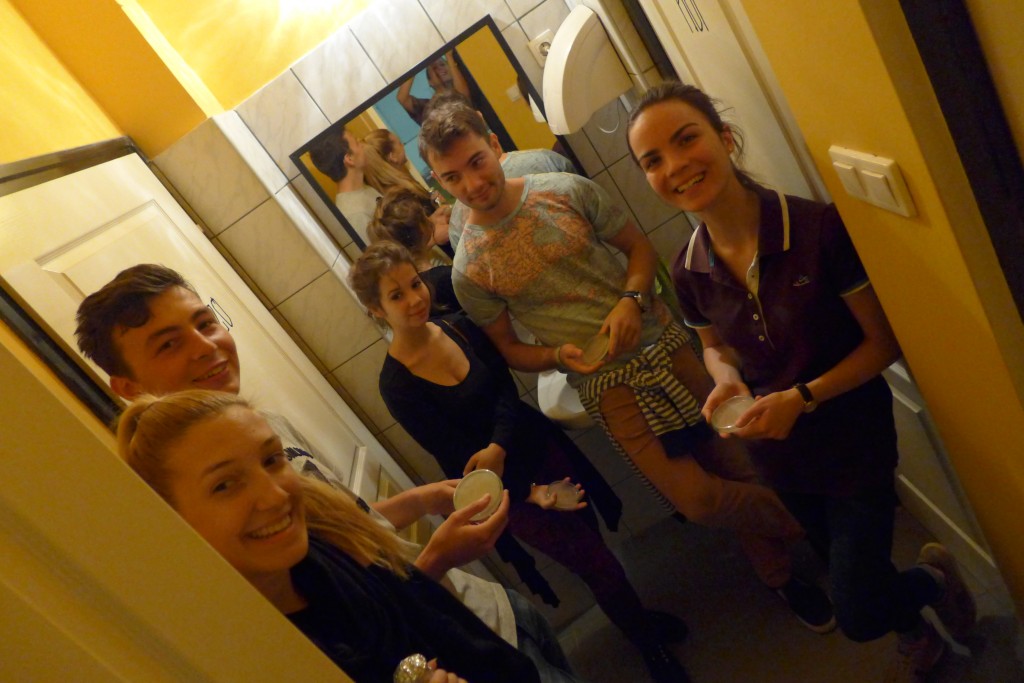Our lab equipment donated to MOME
- Posted by darkeye on May 2nd, 2021 filed in log
- Comment now »
Since the discontinuation of our DIY Bio-Lab at FabLab Budapest, our equipment has been utilized by the Városmajor High School in Budapest, and later they traveled to Maker’s Red Box (formerly known as Makerspace).
Earlier this year, I’ve decided to donate all equipment to the Creative Technology Hub at the Moholy-Nagy Design University (MOME) in Budapest, Hungary.
Post on MOME’s site available here.
on the way to a light source
- Posted by darkeye on August 4th, 2015 filed in experiments, log
- Comment now »
high-school experiments with luminescent bacteria
- Posted by darkeye on August 4th, 2015 filed in events, experiments
- Comment now »
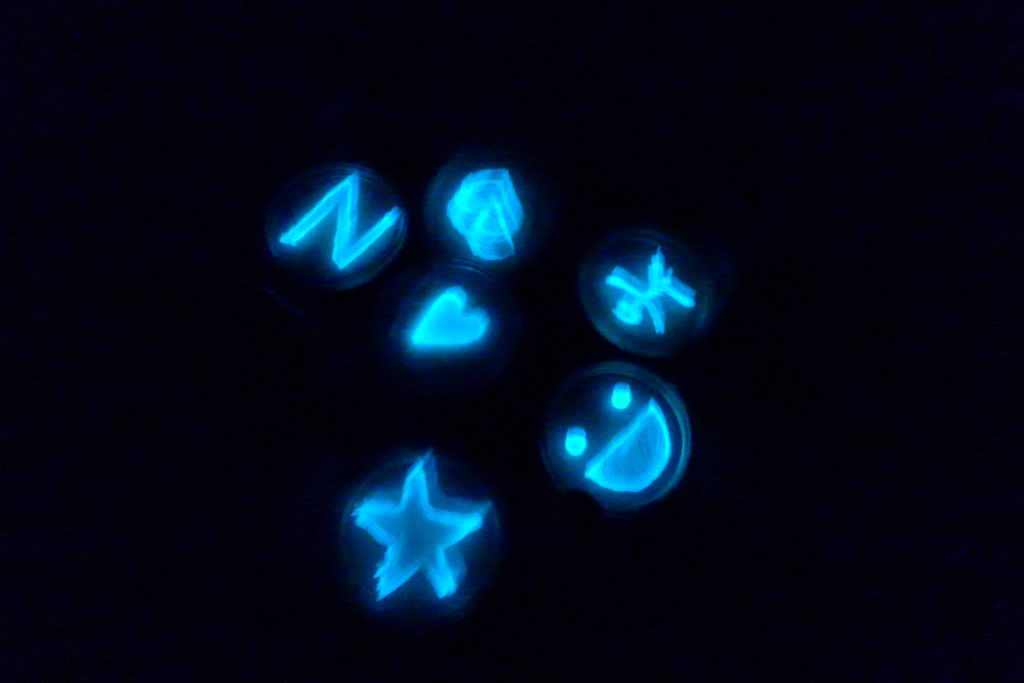
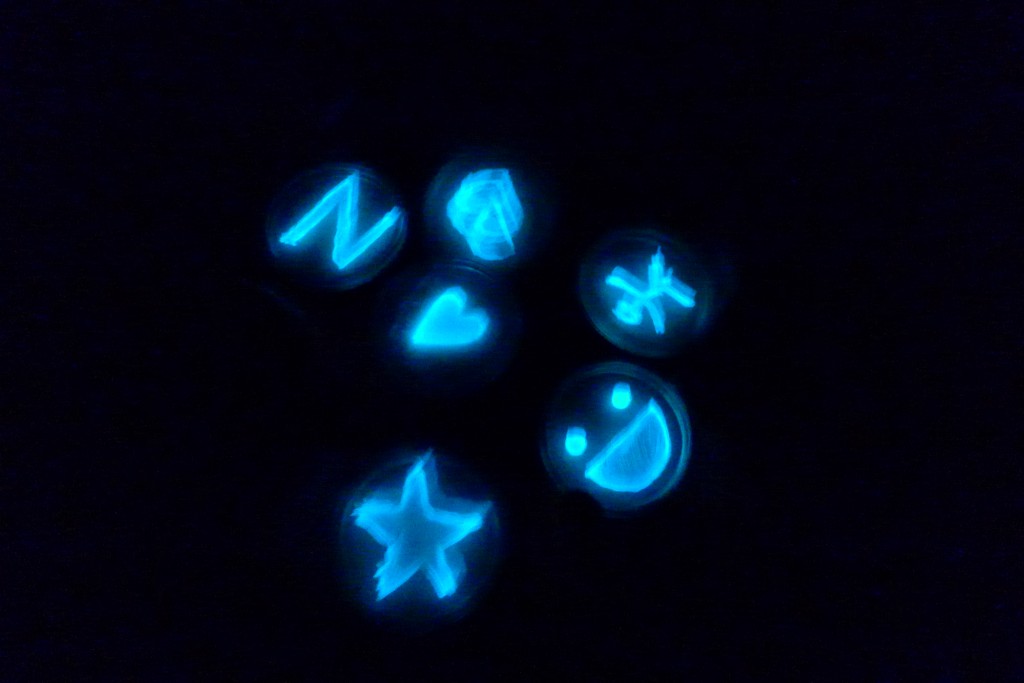
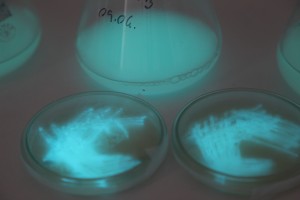
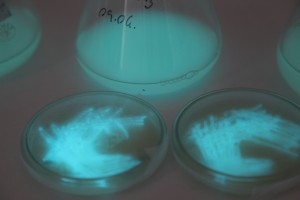
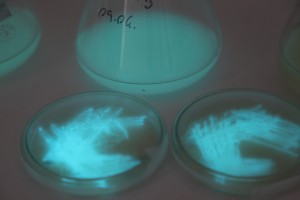
With the help of Margarita Kopniczky of Imperial College London, students from EngAme and theAKG high-school made some happy drawings with Photobacterium Phosphoreum in our small lab. See both the studends and the bacteria smile.
Welcome our smiling bacterial overlords!
- Posted by darkeye on July 30th, 2015 filed in events
- Comment now »
With the help of Margarita Kopniczky of Imperial College London, students from EngAme and the AKG high-school made some happy drawings with Photobacterium Phosphoreum in our small lab.
natural light startup sequence
- Posted by darkeye on July 14th, 2015 filed in experiments
- Comment now »
experiment log 2014.12.21.
- Posted by darkeye on December 21st, 2014 filed in experiments
- Comment now »
yesterday I prepared the following experiments:
- 100ml liquid culture as control in a 500ml Erlenmayer flask, composed of:
- Nutrient Broth: 8g/1000ml -> 0.8g
- NaCl: 30g/1000ml -> 3g
- Glycerol: 10g/1000ml -> 1g
- CaCO3: 5g/1000ml -> 0.5g
- 100ml of the same liquid culture, placed on a magnetic stirrer
- 100ml of the same liquid culture, but without CaCO3
- 100ml of the same liquid culture, but without Glycerol
and left these overnight on the table at room temperature (well, lab temperature, around 20C)
today I’ve found that:
- the control was producing light when shaken
- the culture without the CaCO3 was producing light when shaken
- the culture without Glycerol was producing light when shaken
- the culture on the magnetic stirrer is constantly producing light, with the light visible around the vortex of stirring
thus it seems that a minimalist nutrient liquid without CaCO3 and/or Glycerol might already be sufficient
Photobacterium Phosphoreum in a bottle
- Posted by darkeye on September 7th, 2014 filed in experiments, log
- 1 Comment »
Yesterdays experiment with growing Photobacterium Phosphoreum as a liquid culture came to fruition today. It seems that the ‘botched’ liquid culture from two days ago came to light today as well. The following images show the result, in a pitch dark room with some reflection off the wall (well, a tiled bathroom), at 10sec, f/4, ISO 800:
In short, I prepared two 100ml cultures (one PP BH and one PP LumA) in a 500ml Erlenmayer flask each, with the following composition each:
- Nutrient Broth: 8g/1000ml -> 0.8g
- NaCl: 30g/1000ml -> 3g
- Glycerol: 10g/1000ml -> 1g
- CaCO3: 5g/1000ml -> 0.5g
The ‘botched’ bottle from two days ago had the same ingredients, but in cca. 180ml of water. The result is well visible in a dimly lit room. I personally can’t tell the difference between the PP HB and the PP LumA variants.
The bacteria light up only when you oxygenize the culture, for example, you shake / stir the liquid culture. after that, it stays visibly bright for about 7 minutes. See the following image sequence, taken in a loosely lit room in 1 minute increments, at 3s, f/4, ISO 800:
Photobacterium Phosphoreum
- Posted by darkeye on September 6th, 2014 filed in experiments, log
- 1 Comment »
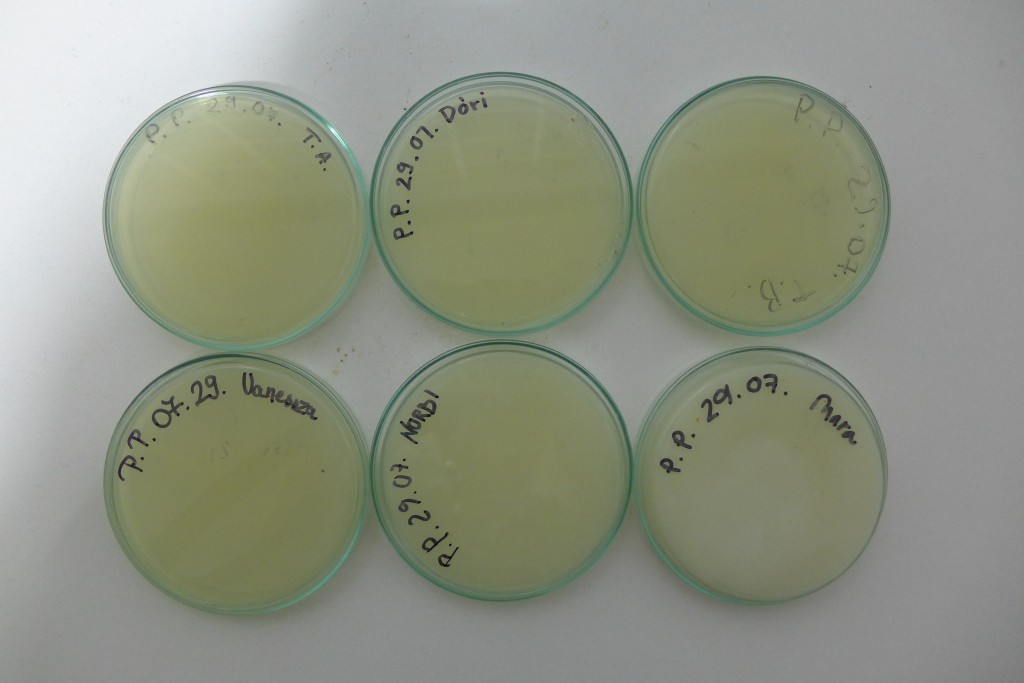
Yesterday I did some experiments with Photbacterium Phosphoreum, received kindly from Simon Park, I received two different batches, one simply labeled PP BH, the other PP LumA. I grew out two of each on agar plates, with the following ingredients for 4 plates, 80ml of Agar total:
- Nutrient Broth: 8g/1000ml -> 0.64g
- NaCl: 30g/1000ml -> 2.4g
- Glycerol: 10g/1000ml -> 0.8g
- CaCO3: 5g/1000ml -> 0.4g
- Agar: 15g/1000ml -> 1.2g
I also tried to create a liquid culture, where I botched the ratios and ended up with twice the liquid for the amount of ingredients.
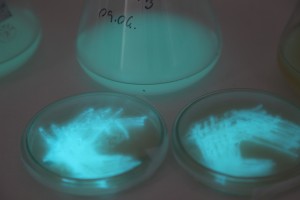
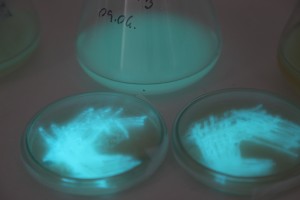
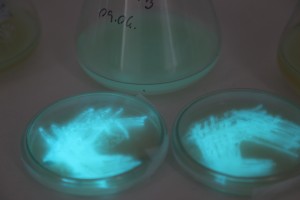
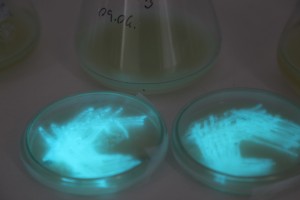
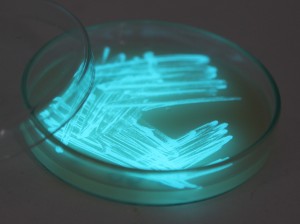
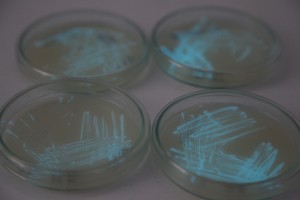
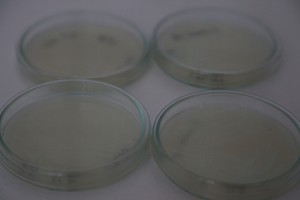
The plates lit up overnight, the liquid culture didn’t. They are quite bright when compared to Vibrio Fischeri. Below are images with various ambient light and photo settings used to take them. other than cropping the first two images, no modification has been made. The two plates on the right left are the PP HB plates, the right ones are the PP LumA plates.
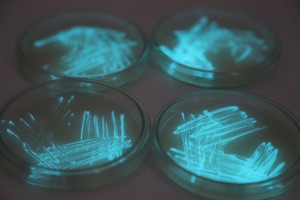
Photobacterium Phosphoreum,
1/2s, f/4, ISO 3200
DNA transformation with friends
- Posted by darkeye on October 21st, 2013 filed in experiments
- Comment now »
last Thursday we performed the first set of DNA transformations with a small circle of friends, Ferenc Szalai, Attila Nemes and András Márton Juhász. this was a basic test of the lab to prove if multiple people can work in it together.
our lab featured at dyibio.org
- Posted by darkeye on October 4th, 2013 filed in related
- Comment now »
 Our lab just got featured at dyibio.org! find is in the local group listings 🙂
Our lab just got featured at dyibio.org! find is in the local group listings 🙂